Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 structure transition under high pressure
structure transition under high pressureYamanaka, Takamitsu*; Nakamoto, Yuki*; Sakata, Masafumi*; Shimizu, Katsuya*; Hattori, Takanori
Physics and Chemistry of Minerals, 51(1), p.4_1 - 4_10, 2024/02
Times Cited Count:0 Percentile:0.34(Materials Science, Multidisciplinary)Neutron and synchrotron X-ray diffraction and electric conductivity measurements of FeTiO ilmenite were performed under pressures. Ilmenite structure is retained up to 28 GPa. Structure analysis revealed that FeO
ilmenite were performed under pressures. Ilmenite structure is retained up to 28 GPa. Structure analysis revealed that FeO and TiO
and TiO are compressible and less compressible below 8 GPa, respectively. The resistivity is lowest along the Fe-Ti direction that has shortest interatomic distance among all the metal ion pairs. The resistivity in the direction normal to c-axis monotonically decreases with pressure, whereas that along c-axis shows hallow-shape with pressure. Maximum entropy analysis shows that electron configuration of Fe
are compressible and less compressible below 8 GPa, respectively. The resistivity is lowest along the Fe-Ti direction that has shortest interatomic distance among all the metal ion pairs. The resistivity in the direction normal to c-axis monotonically decreases with pressure, whereas that along c-axis shows hallow-shape with pressure. Maximum entropy analysis shows that electron configuration of Fe (3
(3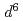 ) is more strongly changed than Ti
) is more strongly changed than Ti (3
(3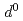 ) under compression. The anisotropic electrical conductivity and non-uniform structure change of Fe-Ti interatomic distance can be explained by the possible spin transition from high-spin state to intermediate-spin state of Fe cation.
) under compression. The anisotropic electrical conductivity and non-uniform structure change of Fe-Ti interatomic distance can be explained by the possible spin transition from high-spin state to intermediate-spin state of Fe cation.
 Fe
Fe O
O solid solutions under high-pressure and high-temperature conditions
solid solutions under high-pressure and high-temperature conditionsYamanaka, Takamitsu*; Hirao, Naohisa*; Nakamoto, Yuki*; Mikouchi, Takashi*; Hattori, Takanori; Komatsu, Kazuki*; Mao, H.-K.*
Physics and Chemistry of Minerals, 49(10), p.41_1 - 41_14, 2022/10
Times Cited Count:0 Percentile:0.02(Materials Science, Multidisciplinary)Magnetic and crystal structure of Mn Fe
Fe O
O solid solutions under high-PT conditions are investigated by neutron diffraction and synchrotron M
solid solutions under high-PT conditions are investigated by neutron diffraction and synchrotron M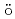 ssbauer spectroscopy. The ferrimagnetic-paramagnetic transition and tetragonal-cubic transition of Mn
ssbauer spectroscopy. The ferrimagnetic-paramagnetic transition and tetragonal-cubic transition of Mn FeO
FeO spinel occur at 100
spinel occur at 100 C and 180
C and 180 C, respectively, suggesting both the transitions are not coupled. The structure transition temperature decreases with pressure. M
C, respectively, suggesting both the transitions are not coupled. The structure transition temperature decreases with pressure. M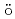 ssbauer experiments and neutron diffraction revealed that the Fe
ssbauer experiments and neutron diffraction revealed that the Fe occupancy in tetrahedral site increases increase with pressure, suggesting Mn
occupancy in tetrahedral site increases increase with pressure, suggesting Mn FeO
FeO phase approaches inverse spinel. Magnetic structure refinement clarified paramagnetic and ferrimagnetic structure of MnFe
phase approaches inverse spinel. Magnetic structure refinement clarified paramagnetic and ferrimagnetic structure of MnFe O
O and Mn
and Mn FeO
FeO . These spinels transform into high-pressure orthorhombic phases at 18.4 and 14.0 GPa, respectively, indicating lower transition pressure with increasing Mn content.
. These spinels transform into high-pressure orthorhombic phases at 18.4 and 14.0 GPa, respectively, indicating lower transition pressure with increasing Mn content.
 and Fe
and Fe distribution in chevkinite-subgroup minerals; X-ray diffraction, neutron diffraction,
distribution in chevkinite-subgroup minerals; X-ray diffraction, neutron diffraction, 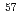 Fe M
Fe M ssbauer spectroscopy and electron-microprobe analyses
ssbauer spectroscopy and electron-microprobe analysesNagashima, Mariko*; Armbruster, T.*; Akasaka, Masahide*; Sano, Asami; Nishio-Hamane, Daisuke*; Malsy, A.*; Imaoka, Teruyoshi*; Nakashima, Kazuo*
Physics and Chemistry of Minerals, 47(6), p.29_1 - 29_18, 2020/06
Times Cited Count:3 Percentile:17.15(Materials Science, Multidisciplinary)Three non-metamict chevkinite-subgroup minerals, from Cape Ashizuri, Japan, Tangir Valley, Diamar District, Pakistan and Haramosh Mts., Skardu district, Pakistan, were studied by crystal chemical techniques. Powder X-ray diffraction and transmission electron microscopic observations confirmed well crystalline samples. Electron-microprobe analyses indicated the general composition known for chevkinite-(Ce). Site scattering values determined by single-crystal X-ray structure refinements suggested assignment of subordinate Nb to the octahedral M3 and M4 sites, minor Th to M1 for the Ashizuri sample and minor Mg to M1 for both samples from Pakistan. Neutron time-of-flight powder diffraction studies were applied to determine the Ti/Fe distribution among octahedral sites for all samples and Mossbauer spectroscopy served for the Fe valence assignment at the four octahedral sites. The dominant iron valence at M1 of the Haramosh sample is ferric whereas for samples Nos. 1 and 2 iron is ferrous.
 Al
Al (O
(O D
D )
) , with temperature at high pressure
, with temperature at high pressureKyono, Atsushi*; Kato, Masato*; Sano, Asami; Machida, Shinichi*; Hattori, Takanori
Physics and Chemistry of Minerals, 46(5), p.459 - 469, 2019/05
Times Cited Count:4 Percentile:20.98(Materials Science, Multidisciplinary)To reveal the decomposition mechanism with temperature under high-pressure, crystal structure of a hydrogrossular, katoite Ca Al
Al (O
(O D
D )
) has been studied by in-situ neutron diffraction at 8 GPa. Although unusual expansion behavior was discerned at 200-400
has been studied by in-situ neutron diffraction at 8 GPa. Although unusual expansion behavior was discerned at 200-400 C, the unit cell was continuously expanded up to 850
C, the unit cell was continuously expanded up to 850 C. At 900
C. At 900 C, katoite was decomposed, indicating that pressure strongly increases dehydration temperature from 300
C, katoite was decomposed, indicating that pressure strongly increases dehydration temperature from 300 C to 900
C to 900 C. On release of pressure, the katoite reappear together with corundum and portlandite. At 8 GPa, CaO
C. On release of pressure, the katoite reappear together with corundum and portlandite. At 8 GPa, CaO and AlO
and AlO polyhedra expand with temperature up to 850
polyhedra expand with temperature up to 850 C by about 8% and 13%, respectively. On the other hand, tetrahedral interstices are isotopically squeezed by about 10%: due to the expansion of above polyhedra. The neighboring D-D distance remains almost unchanged in this temperature range, while the O-D bond distance shrinks drastically just before decomposition. This finding suggests that the shortening of O-D distance caused by the D-D repulsion destabilizes the O-D bond, which induces the thermal decomposition of katoite.
C by about 8% and 13%, respectively. On the other hand, tetrahedral interstices are isotopically squeezed by about 10%: due to the expansion of above polyhedra. The neighboring D-D distance remains almost unchanged in this temperature range, while the O-D bond distance shrinks drastically just before decomposition. This finding suggests that the shortening of O-D distance caused by the D-D repulsion destabilizes the O-D bond, which induces the thermal decomposition of katoite.
Tomioka, Naotaka*; Okuchi, Takuo*; Purevjav, N.*; Abe, Jun*; Harjo, S.
Physics and Chemistry of Minerals, 43(4), p.267 - 275, 2016/04
Times Cited Count:8 Percentile:28.75(Materials Science, Multidisciplinary) -AlOOH by using single-crystal synchrotron X-ray diffraction method
-AlOOH by using single-crystal synchrotron X-ray diffraction methodKuribayashi, Takahiro*; Sano, Asami; Nagase, Toshiro*
Physics and Chemistry of Minerals, 41(4), p.303 - 312, 2014/04
Times Cited Count:39 Percentile:79.07(Materials Science, Multidisciplinary)Pressure-induced phase transition of  -AlOOH was confirmed between 6.1 and 8.2 GPa by using a single-crystal synchrotron X-ray diffraction method. The phase transition is reversible and unquenchable. The space group of the post-transition phase should be
-AlOOH was confirmed between 6.1 and 8.2 GPa by using a single-crystal synchrotron X-ray diffraction method. The phase transition is reversible and unquenchable. The space group of the post-transition phase should be 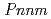 or
or 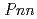 2 to satisfy the systematic absence rule. Crystal structure refinements of the post-transition phase conducted for the three models (
2 to satisfy the systematic absence rule. Crystal structure refinements of the post-transition phase conducted for the three models (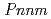 ,
, 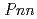 2, and
2, and  2
2
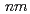 ) indicate that the space group of the post-transition phase is
) indicate that the space group of the post-transition phase is 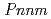 . The O-O distance of hydrogen bond in the post-transition phase at 8.2 GPa is 2.439(6)
. The O-O distance of hydrogen bond in the post-transition phase at 8.2 GPa is 2.439(6)  and is significantly longer than the predicted distance (2.366
and is significantly longer than the predicted distance (2.366  ) of the hydrogen bond symmetrization in
) of the hydrogen bond symmetrization in  -AlOOH. The H distribution in the post-transition phase would display a fully disordered hydrogen bond pattern.
-AlOOH. The H distribution in the post-transition phase would display a fully disordered hydrogen bond pattern.
Sano, Asami; Yagi, Takehiko*; Okada, Taku*; Goto, Hirotada*; Kikegawa, Takumi*
Physics and Chemistry of Minerals, 39(5), p.375 - 383, 2012/05
Times Cited Count:20 Percentile:55.17(Materials Science, Multidisciplinary)X-ray diffraction measurements of distorted rutile-type oxyhydroxides  -GaOOH, InOOH,
-GaOOH, InOOH,  -CrOOH, and
-CrOOH, and  -CrOOD were taken at a maximum pressure of up to 35 GPa under quasi-hydrostatic conditions, at ambient temperature. Anomalies in the evolution of the relative lattice constants and the axial ratios of
-CrOOD were taken at a maximum pressure of up to 35 GPa under quasi-hydrostatic conditions, at ambient temperature. Anomalies in the evolution of the relative lattice constants and the axial ratios of  -GaOOH, InOOH, and
-GaOOH, InOOH, and  -CrOOD suggest anisotropic stiffening along the a- and/or b-axes where the hydrogen bond is formed. The changes were observed at 15 GPa in
-CrOOD suggest anisotropic stiffening along the a- and/or b-axes where the hydrogen bond is formed. The changes were observed at 15 GPa in  -GaOOH and InOOH and at 4 GPa in
-GaOOH and InOOH and at 4 GPa in  -CrOOD. The pressures were higher in oxyhydroxides that have longer O...O distances of the hydrogen bond at ambient pressure. In contrast, such stiffening behavior was not observed in CrOOH, which has a significant short O...O distance and strong hydrogen bond. The stiffening behaviors observed in the present study can be attributed to the symmetrization of the hydrogen bonds in oxyhydroxides, as was previously found in
-CrOOD. The pressures were higher in oxyhydroxides that have longer O...O distances of the hydrogen bond at ambient pressure. In contrast, such stiffening behavior was not observed in CrOOH, which has a significant short O...O distance and strong hydrogen bond. The stiffening behaviors observed in the present study can be attributed to the symmetrization of the hydrogen bonds in oxyhydroxides, as was previously found in  -AlOOH(D).
-AlOOH(D).
Iizuka, Riko*; Kagi, Hiroyuki*; Komatsu, Kazuki*; Ushijima, Daichi*; Nakano, Satoshi*; Sano, Asami; Nagai, Takaya*; Yagi, Takehiko*
Physics and Chemistry of Minerals, 38(10), p.777 - 785, 2011/12
Times Cited Count:10 Percentile:33.32(Materials Science, Multidisciplinary)The pressure responses of portlandite and the isotope effect on the phase transition were investigated at room temperature from single-crystal Raman and IR spectra and from powder X-ray diffraction using diamond anvil cells under quasi-hydrostatic conditions in a helium pressure-transmitting medium. Phase transformation and subsequent peak broadening observed from the Raman and IR spectra of Ca(OH) occurred at lower pressures than those of Ca(OD)
occurred at lower pressures than those of Ca(OD) . In contrast, no isotope effect was found on the volume and axial compressions observed from powder X-ray diffraction patterns. X-ray diffraction lines attributable to the high-pressure phase remained up to 28.5 GPa, suggesting no total amorphization in a helium pressure medium within the examined pressure region. These results suggest that the H-D isotope effect is engendered in the local environment surrounding H(D) atoms.
. In contrast, no isotope effect was found on the volume and axial compressions observed from powder X-ray diffraction patterns. X-ray diffraction lines attributable to the high-pressure phase remained up to 28.5 GPa, suggesting no total amorphization in a helium pressure medium within the examined pressure region. These results suggest that the H-D isotope effect is engendered in the local environment surrounding H(D) atoms.
Okada, Taku; Utsumi, Wataru; Kaneko, Hiroshi*; Turkevich, V.*; Hamaya, Nozomu; Shimomura, Osamu*
Physics and Chemistry of Minerals, 31(4), p.261 - 268, 2004/05
Times Cited Count:12 Percentile:41.33(Materials Science, Multidisciplinary)The graphite-diamond transformation was investigated by in situ time-resolved X-ray diffraction experiments using aqueous fluid containing dissolved MgO as the diamond forming catalyst under conditions of 6.6-8.9 GPa and 1400-1835  C. The transformed volume fractions of diamond as a function of time under various pressure-temperature conditions were obtained and analyzed using the JMAK rate equation. Variations in the nucleation and growth processes during diamond formation as a function of pressure and temperature were clarified.
C. The transformed volume fractions of diamond as a function of time under various pressure-temperature conditions were obtained and analyzed using the JMAK rate equation. Variations in the nucleation and growth processes during diamond formation as a function of pressure and temperature were clarified.
Okada, Taku; Utsumi, Wataru; Kaneko, Hiroshi*; Yamakata, Masaaki*; Shimomura, Osamu
Physics and Chemistry of Minerals, 29(7), p.439 - 445, 2002/08
Times Cited Count:18 Percentile:53.64(Materials Science, Multidisciplinary)An experimental technique to make real-time observations at high pressure and temperature of the diamond forming process in candidate material of mantle fluids as a catalyst has been established for the first time. In situ X-ray diffraction experiments using synchrotron radiation have been performed upon a mixture of brucite (Mg(OH) ) and graphite as starting material. Brucite decomposes into periclase (MgO) and H
) and graphite as starting material. Brucite decomposes into periclase (MgO) and H O at 3.6 GPa and 1050
O at 3.6 GPa and 1050 C while no periclase is formed after the decomposition of brucite at 6.2 GPa and 1150
C while no periclase is formed after the decomposition of brucite at 6.2 GPa and 1150 C, indicating that the solubility of the MgO component in H
C, indicating that the solubility of the MgO component in H O greatly increases with increasing pressure. The conversion of graphite to diamond in aqueous fluid has been observed at 7.7 GPa and 1835
O greatly increases with increasing pressure. The conversion of graphite to diamond in aqueous fluid has been observed at 7.7 GPa and 1835 C. Time-dependent X-ray diffraction profiles for this transformation have been successfully obtained.
C. Time-dependent X-ray diffraction profiles for this transformation have been successfully obtained.
 determined by in situ X-ray observations up to 30GPa and 1400K
determined by in situ X-ray observations up to 30GPa and 1400KOno, Shigeaki*; Ito, Eiji*; Katsura, Tomoo*; Yoneda, Akira*; Walter, M.*; Urakawa, Satoru*; Utsumi, Wataru; Funakoshi, Kenichi*
Physics and Chemistry of Minerals, 27(9), p.618 - 622, 2000/11
Times Cited Count:44 Percentile:79.82(Materials Science, Multidisciplinary)no abstracts in English
 by in situ X-ray diffraction and quench experiments
by in situ X-ray diffraction and quench experimentsKuroda, Koji*; Irifune, Tetsuo*; Inoue, Toru*; Nishiyama, Norimasa*; Miyashita, Minoru*; Funakoshi, Kenichi*; Utsumi, Wataru
Physics and Chemistry of Minerals, 27(8), p.523 - 532, 2000/09
Times Cited Count:38 Percentile:76.41(Materials Science, Multidisciplinary)no abstracts in English
Fukui, Hiroyuki*; Otaka, Osamu*; Nagai, Takaya*; Katsura, Tomoo*; Funakoshi, Kenichi*; Utsumi, Wataru
Physics and Chemistry of Minerals, 27(6), p.367 - 370, 2000/06
Times Cited Count:4 Percentile:21.55(Materials Science, Multidisciplinary)no abstracts in English
 O
O
Urakawa, Satoru*; Kondo, Tadashi*; Igawa, Naoki; Shimomura, Osamu*; Ono, Hideo
Physics and Chemistry of Minerals, 21, p.387 - 391, 1994/00
no abstracts in English